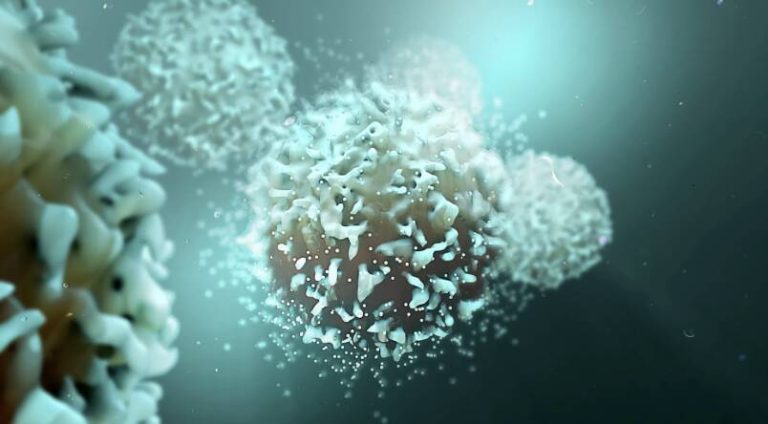
In a first, gene-edited cell therapy cures aggressive blood cancer
In a groundbreaking medical breakthrough, UK researchers have successfully reversed T-cell acute lymphoblastic leukaemia, an aggressive blood cancer, using gene-edited immune cells for the first time. This innovative therapy, called BE-CAR7, modifies immune cells (T-cells) to have chimeric antigen receptors (CARs) on their surface. The CARs recognize and target a specific protein on cancer cells’ surface, and the T-cell attached then destroys that cancer cell. This pioneering approach has opened up new avenues for the treatment of blood cancers, offering hope to patients who have limited treatment options.
T-cell acute lymphoblastic leukaemia (T-ALL) is a type of blood cancer that affects the T-cells, a crucial component of the immune system. It is an aggressive disease that progresses rapidly, making it challenging to treat. Current treatments for T-ALL often involve chemotherapy, radiation, and bone marrow transplantation, which can have severe side effects and may not always be effective. The development of gene-edited cell therapy has the potential to revolutionize the treatment of this disease, providing a more targeted and effective approach.
The BE-CAR7 therapy involves extracting T-cells from the patient’s blood and modifying them using gene editing techniques. The modified T-cells are then infused back into the patient, where they can recognize and target cancer cells. The CARs on the surface of the T-cells are designed to recognize a specific protein called CD7, which is present on the surface of T-ALL cancer cells. When the modified T-cells encounter cancer cells with CD7, they become activated and destroy the cancer cells.
The success of the BE-CAR7 therapy is a significant milestone in the development of gene-edited cell therapies for cancer treatment. Previous studies have shown promising results using similar approaches, but this is the first time that gene-edited immune cells have been used to cure an aggressive blood cancer. The study demonstrates the potential of gene editing to create targeted and effective treatments for a range of diseases, including cancer.
The use of gene-edited cell therapy has several advantages over traditional cancer treatments. It is a highly targeted approach, which reduces the risk of harm to healthy cells. The modified T-cells can also persist in the body for an extended period, providing long-term protection against cancer. Additionally, the therapy can be tailored to the individual patient, using their own T-cells to create a personalized treatment.
The development of the BE-CAR7 therapy is a testament to the power of medical research and innovation. The study was conducted by a team of researchers from the UK, who used cutting-edge gene editing techniques to modify the T-cells. The results of the study have been published in a leading medical journal, providing a detailed account of the therapy and its effectiveness.
The implications of this breakthrough are significant, and it is likely to have a major impact on the treatment of blood cancers. The BE-CAR7 therapy has the potential to be used in combination with other treatments, such as chemotherapy and radiation, to create a comprehensive treatment plan. It may also be used to treat other types of blood cancers, including B-cell acute lymphoblastic leukaemia and chronic lymphocytic leukaemia.
In conclusion, the success of the BE-CAR7 therapy is a major breakthrough in the treatment of aggressive blood cancer. The use of gene-edited immune cells to target and destroy cancer cells is a highly effective and targeted approach. The study demonstrates the potential of gene editing to create innovative treatments for a range of diseases, including cancer. As research continues to advance, it is likely that we will see the development of new and innovative therapies that can provide hope to patients with limited treatment options.
For more information on this groundbreaking study, please visit: https://www.sciencedaily.com/releases/2025/12/251211040438.htm
News Source: https://www.sciencedaily.com/releases/2025/12/251211040438.htm