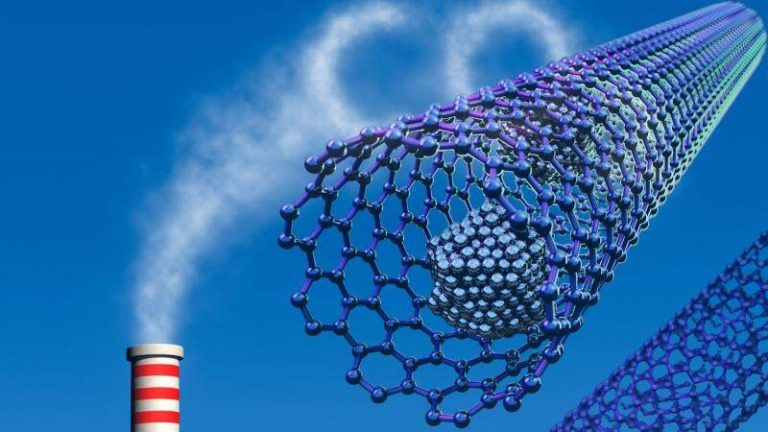
Safer method boosts gas capture for clean energy
The world is shifting towards cleaner energy sources to combat climate change, and one crucial aspect of this transition is the capture and storage of greenhouse gases. Metal-organic frameworks (MOFs) have emerged as a promising material for this purpose, with their high surface area and tunable properties making them ideal for trapping carbon dioxide and other gases. However, the traditional synthesis methods for MOFs often involve the use of toxic hydrofluoric acid, which poses significant environmental and health risks. In a breakthrough development, researchers have now discovered a safer and more efficient method for synthesizing MOFs, paving the way for the widespread adoption of these materials in carbon capture and storage applications.
The new method, which replaces hydrofluoric acid with safer modulators, has been shown to produce superior MOF crystals that can trap greenhouse gases and store hydrogen more efficiently at room temperature. This is a significant improvement over traditional methods, which often require high temperatures and pressures to achieve the same level of gas capture. The simplified synthesis process also makes it easier to scale up production, reducing the cost and environmental impact of MOF manufacturing.
The implications of this breakthrough are far-reaching, with potential applications in a wide range of fields. One of the most significant benefits is the development of affordable carbon scrubbers, which can be used to remove carbon dioxide from power plant emissions and other industrial sources. By capturing and storing CO2, these scrubbers can help reduce the amount of greenhouse gases in the atmosphere, slowing the rate of climate change. Additionally, the advanced MOFs can be used to harvest water from the air, even in arid environments, providing a sustainable source of clean drinking water for communities around the world.
The traditional synthesis method for MOFs involves the use of hydrofluoric acid, a highly toxic and corrosive substance that requires specialized handling and storage. The acid is used to create a solvent that helps to form the MOF crystals, but it also poses significant environmental and health risks. In contrast, the new method uses safer modulators that are more environmentally friendly and can be easily disposed of. This not only reduces the risk of accidents and exposure but also makes the synthesis process more sustainable and cost-effective.
The researchers behind the breakthrough have demonstrated the effectiveness of their new method by synthesizing a range of MOFs with different properties and structures. They have shown that the resulting crystals have a higher surface area and better gas capture capabilities than those produced using traditional methods. The MOFs have also been tested for their ability to store hydrogen, which is a key component of many clean energy applications. The results have been impressive, with the MOFs demonstrating a higher hydrogen storage capacity than many other materials currently in use.
The development of safer and more efficient MOF synthesis methods is a significant step forward in the quest for clean energy. As the world continues to transition away from fossil fuels and towards renewable energy sources, the demand for advanced materials like MOFs is likely to grow. By providing a more sustainable and cost-effective way to produce these materials, the researchers have paved the way for the widespread adoption of MOFs in a range of applications, from carbon capture and storage to hydrogen fuel cells and beyond.
In addition to their potential for carbon capture and storage, MOFs also have a range of other applications in fields such as catalysis, drug delivery, and sensing. Their high surface area and tunable properties make them ideal for a wide range of tasks, from catalyzing chemical reactions to detecting specific molecules. As the synthesis methods continue to improve, it is likely that we will see MOFs being used in an increasingly diverse range of applications, from consumer products to industrial processes.
The breakthrough in MOF synthesis is also significant because it demonstrates the importance of interdisciplinary research in driving innovation. The team behind the discovery brought together experts from a range of fields, including chemistry, materials science, and engineering. By combining their knowledge and expertise, they were able to develop a new method that overcomes many of the limitations of traditional MOF synthesis. This collaborative approach is essential for tackling complex problems like climate change, where a range of different disciplines and expertise are required to develop effective solutions.
In conclusion, the development of a safer and more efficient method for synthesizing metal-organic frameworks is a significant breakthrough in the quest for clean energy. By replacing toxic hydrofluoric acid with safer modulators, the researchers have paved the way for the widespread adoption of MOFs in carbon capture and storage applications. The potential implications of this discovery are far-reaching, with potential applications in a range of fields from carbon scrubbers to hydrogen fuel cells. As the world continues to transition towards cleaner energy sources, the demand for advanced materials like MOFs is likely to grow, and this breakthrough is an important step forward in meeting that demand.
News Source: https://researchmatters.in/news/greener-path-synthesising-metal-organic-frameworks-carbon-capture-and-storage