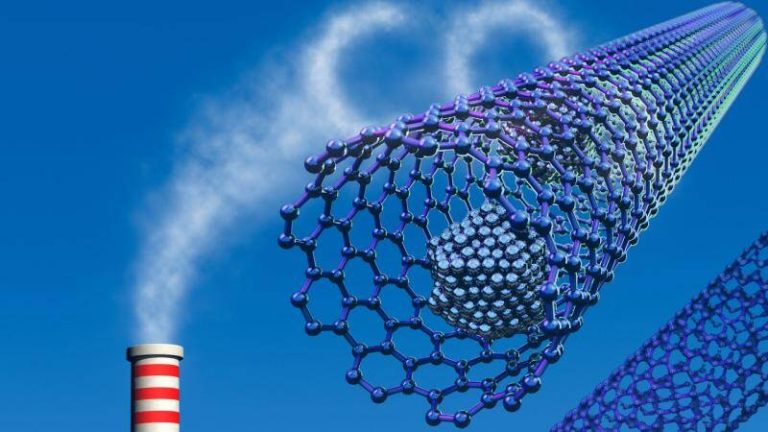
Safer method boosts gas capture for clean energy
The world is grappling with the challenges of climate change, and one of the most significant contributors to this problem is the increasing levels of greenhouse gases in the atmosphere. Carbon dioxide, in particular, is a major culprit, and finding effective ways to capture and store it is crucial for mitigating its impact. Researchers have been exploring various methods to achieve this, and a recent breakthrough in the synthesis of metal-organic frameworks (MOFs) promises to revolutionize the field of carbon capture and storage.
Traditionally, the synthesis of MOFs has involved the use of hydrofluoric acid, a highly toxic and corrosive substance that poses significant risks to human health and the environment. However, a team of researchers has developed a fluoride-free synthesis method that replaces hydrofluoric acid with safer modulators. This innovative approach not only reduces the risks associated with MOF synthesis but also produces superior crystals that are more efficient at trapping greenhouse gases and storing hydrogen at room temperature.
The new method is a significant improvement over existing techniques, as it simplifies the synthesis process and eliminates the need for toxic chemicals. The resulting MOFs have a more uniform structure and larger surface area, which enables them to capture and store gases more efficiently. This breakthrough has far-reaching implications for the development of affordable carbon scrubbers and advanced atmospheric water harvesting systems, which are essential for fighting climate change globally.
One of the most significant advantages of the new synthesis method is its ability to produce MOFs that can capture carbon dioxide at room temperature. This is a major breakthrough, as most existing carbon capture technologies require high temperatures and pressures, which are energy-intensive and expensive. The new MOFs, on the other hand, can capture carbon dioxide at ambient temperatures, making them ideal for use in a wide range of applications, from industrial processes to vehicle emissions.
The potential applications of the new MOFs are vast and varied. For example, they could be used to develop more efficient carbon scrubbers for power plants, reducing the amount of greenhouse gases released into the atmosphere. They could also be used to create advanced atmospheric water harvesting systems, which could provide clean drinking water for millions of people around the world. Additionally, the MOFs could be used to store hydrogen, which is a clean and efficient fuel source that could power vehicles and other applications.
The development of the new synthesis method is a testament to the power of innovative research and its potential to drive positive change. By replacing toxic chemicals with safer modulators, the researchers have created a more sustainable and environmentally friendly method for producing MOFs. This not only reduces the risks associated with MOF synthesis but also paves the way for the widespread adoption of these materials in a variety of applications.
The impact of this breakthrough could be significant, particularly in the context of climate change. By providing a more efficient and cost-effective way to capture and store greenhouse gases, the new MOFs could play a major role in reducing the amount of carbon dioxide in the atmosphere. This, in turn, could help to mitigate the effects of climate change, from rising sea levels to more frequent natural disasters.
In conclusion, the development of a fluoride-free synthesis method for metal-organic frameworks is a major breakthrough that promises to revolutionize the field of carbon capture and storage. By producing superior crystals that can trap greenhouse gases and store hydrogen more efficiently at room temperature, the new MOFs have the potential to drive positive change and help combat climate change. As researchers continue to explore the potential applications of these materials, it is clear that the future of clean energy is looking brighter than ever.